|

Dear Robert
Thank you for the Charles 803 Alcohol Fuel Still plans they arrived on the 18th of January. They are superb, excellent
detail. I will definitely let you know how I get on with its construction, although probably not for a
little while.
Many thanks, John
Dear Robert,
Hi, My name is Ted. I'm sort of Mark's partner in alternative
energy crime. Hope this check is sufficient for the magic Penn valve.
I've got almost all of the parts collected for the still.,
save for the ¼ inch coil. I have near to 300 gallons of mash ready to run.
My family has made whiskey since I was a kid, so we have a
bit of experience. My cousin works at a local pie/pastry factory & brings me ample supplys of sugar (5 or 600 pounds,
so far.) I fermented 35 five-gallon buckets of apple pie filling & recently
a light truckload of frozen fruit that was slightly freezer burnt.
We're also developing wood gasification. Mark may or may
not have told you about it. I've been off grid for 27 years and now my goal is to be off petroleum: veggie based motor oil
and all.
Thanks again,
Ted.
Dear Mark and Ted,
I got your letter and check this morning.
The automatic still valve will be in the post later today. It sounds
like you need to get started soon, or you will be up to your neck in fruit flies, and then your mash starts to turn sour.
In hot weather, there is always the danger of good mash getting contaminated by fruit flies, which carry bacteria which starts
the degradation of sugar into acetic acid (vinegar). Keep it covered up. This is the reason for fermentation locks. They are
cheap, and can be plugged into a small hole in the lid of your 5 gallon buckets, which is a lot cleaner than leaving the lid
ajar.
So,
get busy, build the still, and once you get the valve, then start cooking. Sounds like you're raring to go!
Also, if you are
still interested in sorghum, check out my web page section on molasses, as someone like you can make fuel for practically
free.
Good luck,
Robert Warren
Robert,
> Just a couple of questions.
> 1. How much ethanol from a ton of corn?
The average is 2.6 gallons pure alcohol per bushel (56 pounds). This works out to (2.6 gal. x 7.34 lbs/gal)/56 pounds
= 0.34 gal/lb. multiply by 2,000 pounds (1 ton) = 681 gallons per ton. The big plants producing 40 million gallons per year
(and selling it to the petroleum industry to make unleaded premium) are getting more like 2.8 to 2.9 gallons per bushel, as
they use special yeasts and enzymes to maximize starch conversion.
At these really big plants, including the price of corn and distillation costs, they are making ethanol for around $1.20
to $1.60/ gallon (according to 2004 reports at the Farm Ethanol Workshop in Madison, WI on June 23rd. 2004). (The price of
corn varies from $2.30/bushel to $3.30/bushel in a single year). Ethanol is just one of three products that they get from
the corn: the others are distillers dried grain (DDG), which is about 25% protein, and they also sell CO2 to the beverage
industry.
The big commercial alcohol distilleries throughout the US, in the Midwest, are using dry corn, as it weighs less dry,
so is easier to transport and store large quantities at the distillation facility.
> 2. How much labor to run this still? How automated can you make one?
It can be a lot of work, but you can use front end loaders and various farm machinery to do most of the work. This all
depends on your skill level and how much equipment you have or buy. Use a pump to move your mash and to stir it.
>
> 3. What is the moisture content of the grain after it comes out of the still?
It comes out wet, but you can dry it in the sun. Cows and pigs like to eat it wet. In the big mills, they centrifuge it
so it can by dried and stored, but they prefer to sell it wet as drying uses a lot of expensive natural gas.
> 4. How big of a building do I need to house a Still?
I used to run one in a spare bedroom with the windows open because of the open flame and CO2, etc. But a garage or barn
is better.
Robert,
I received your email with the instructions in .pdf format.
Today I received an envelope with (1) blueprint.
I have already started pricing the components and finding the feedstock.
I have the opportunity to get 1300 gal of waste molasses from a feed mill, in return for getting it out of their
obsolete tank.
I also saw the opportunity to collect wasted corn meal, oats, wheat, and other grains that leak out of cracks in the
hammer mills and fall on the floor.
The powder piles up 2ft deep in about a month.
I'm thinking of offering to clean up the mill once a month for $200 and I get to keep the waste.
I'm also looking for damaged or "off-taste" soda pop syrup tanks from local restaurants too.
Thanks,
Mike Powers
>Hi Robert,
>I'm reviewing still designs in preparation for making a consumption
still. I have read your web articles and comments.
>Since the 803 is a continuous still I see a problem
for consumption in that the heads will be mixed in
with the ethanol. Not a problem for fuel but a big
headache for consumers. Here are my questions:
>Would you recommend a separate pass through your still
with a different temperature setting to distill off
the methanol and leave the ethanol behind?
Dave, First of all you are wrong in assuming it is a continuous still. Look at the diagram
on the enclose diagram. You can clearly see that it is a batch still, it is just that I have separated the boiler from the
reflux column so we can control the flow of steam. Secondly, the methanol is removed by dissolving it into the doubler or
thumper, which is a built-in feature at the bottom interior of the still.
Finally, if you are after a drinking whiskey
still, there are a lot of smaller and cheaper stills you can build. While this still does a pretty good job of removing
the methanol and fusil oils, I don't drink the output. It looks as clear as water, and there are no floating oils at the top,
so it seems to be pretty clear. I have sold a few of my still plans to folks wanting drinking alcohol, but generally
these were for either small commercial production or for laboratory testing. --Robert W.
From: "Mario
Subject: Re: alcohol still inquiry
hi Robert
Does the blueprints have all the measurements of all the parts in the still etc. So all I basically have to do is get
the parts and put it together, is that correct?
My planned feedstock will either be fodder beets or sugar beets at this stage. Looking into other things as well. Scientists
here have just 'created' a genetically modified bush...cant remember name right now but can get it for you if you like which
produces very high sugar levels and once planted can give 3 years good production b4 needing to be replanted etc. sounds very
promising ..Field trials starting soon. Just a pity its genetically modified!-Mario
Mario,
Yes, all the measurements and instructions are quite accurate and detailed. I also provide instruction on soldering copper
pipe, if you have not done this before. But this is not like screwing together wood screws from an Ikea furniture box. This
requires some knowledge of working with tools, a torch, and electric drill and bits, etc. Plus you also have to make a decision
about whether or not to do a conversion on a car you already have, or perhaps to experiment on something less expensive first,
like a lawnmower or small motorbike. I dont recommend doing a conversion on your own car unless you know a bit about this,
or work with someone else who does.
But the sugar beets are a great source for fuel. Your will need a big grinder, such as a hammer mill to grind it first.
Also, you need to have a place to put the large amount of compost you will create, or else it will smell quite bad.
This project better suits a farmer, and isn't for the average city apartment dweller.
Robert Warren
Weds Nov. 21, 2001
Dear Robert
I had a look at the archives of the Biofuels mailing list on Yahoo (Keith Addison
suggested this) and I still think he did a hatchet job on you. Basically, the story Addison tells, as interpreted by me, goes
like this:
1. He bought your still and plans and your old papers for scanning.
2. He found some old Tallgrass literature
in there.
3. He jumped to (unwarranted) conclusion that Charles 803 = plagiarized Tallgrass
4. He dug up some old
derogatory info on Tallgrass still. 5. QED, says Addison, Charles 803 doesn't work.
6. He asks Mike Nixon to look it over.
Nixon is an engineer who co-wrote a 'book ' (more like a pamphlet) on vodka making. His technology is highly conventional
and geared to production of potable spirits. Nixon crapped on the Charles 803, deeming it unsafe (for no good reason I can
see) and unsuitable for making liquor -- a purpose for which it was never intended. He ridicules the bubbler/doubler as worthless
without mentioning that this device, often called a 'keg thumper' is an old moonshiner's trick, known on 3-4 continents.
7.
With Nixon's highly off-base critique to back him up, Addison tossed your work off the j.t.f. site. He implies you are a plagiarist,
indicts you for aggravated capitalism and mopery, publishes sources for the auto valve other than you, then claims auto valve
is not needed at all, but it merely a luxury. What an asshole! Problems are:
a. Tallgrass does not equal Charles 803, even a cursory examination reveals that Tallgrass's stripper coil is
above, not within, the packing, and is smaller than the condenser coil, both are reverse of Charles 803. Tallgrass does not
incorporate the back to back reducers ('alternative method') used to connect but partially thermally decouple the condenser
section from the reflux section. The Tallgrass bubbler section lacks the baffle spool/plate and mesh packing of the Charles
803.
b. You (and Pete Charles) never intended this as a liquor still. You said so loud and clear and even said why, in
several ways.
c. Addison is welcome to regard your instructions as not well written, but, that's neither a crime nor uncommon.
d. Overly criticizing you for not adding whistles and bells like pressure relief valves flies in the face of your obvious
intent to keep the Charles 803 as simple as possible. Industrial pressure relief valves are cheap and anyway much the same
result can be achieved with something as simple as a brass funnel with a ball bearing sitting in it, mounted in the top cap
of the column. If (read when) the boiler surges, overloading or flooding the column, the pressure will push the bearing up,
drop to 1 atmosphere again, and that is that.
e. Calling your still a pipe bomb is ridiculous. I have plans sitting here for 100 gallon commercial liquor stills
and their columns do not have open tops. Mike Nixon's plans for a fractionating still, which he sells online, do not involve
an open top column. Physician, heal thyself. All that being said, the Charles 803 is not a fractionating still, for the simple
reason that it does not include a "reflux head" which selectively directs all or part of the condensate at still head either
back to the column or to the receiver. Automatic reflex heads are available for a pilot plant scale still similar in size
to Charles 803, but they are expensive ($2500) and are really intended to be used in conjunction with vacuum jacketed or otherwise
insulated fractionating columns, which in a 1 to 4 inch ID and 20 to 60 inch working height, can cost thousands of dollars
more. Furthermore, the energy costs of a high reflux rate distillation are enormous -- after all, one is sending 90 to 95%
of the condensate back to the column for enrichment, so the process takes 10-20 times longer and consumes 10-20 times the
energy (plus losses). WHY AM I INTERESTED IN CHARLES 803? Because if as claimed it can strip out 5 gallons of 80-85-90% (not
95%) ethanol from mash, it is useful to me, even though (NOT if) I must (MUST!) post process the ethanol to remove congeners
(methanol, fusels, isopropyl alcohol, acetone). This is because I can build this thing for <$500, while a 100 gallon commercial
liquor still costs $35,000 FOB Kentucky. Or the other favourite device I use costs $15,000 to $20,000 on a scale that produces
2.5 gallons an hour, of 30-40%, or 6 litres an hour for a larger model that costs $40,000. In every case I always have to
post process the alcohol extensively to obtain potable ethanol to the standards I require. No still produces decent liquor
in a single pass. Pot stills are used to redistill liquor 2-3-4 times and at every pass, 'heads' and 'tails' are removed and
discarded (or maybe retained for more processing) and this is done by temperature. Fractionating stills like Mike Nixon loves,
might produce alcohol that is drinkable, technically, but I can assure you it won't be FUN to drink unless it is carefully
polished with carbon, a process that is not as simple as people would have you believe. Doing this right involves first fractionating
to 95%, then diluting to 40-50%, then running through carefully prepared columns of granulated AC, of particular grades, at
particular rates, and QCing what comes out by gas chromatography, usually Solid Phase Micro-Extraction these days, because
it is (relatively fast and easy compared to say, Liquid Extraction and then evaporative concentration -- this means extracing
with Freon 11--a 4 day process and somewhat hazardous. Anyway, I think you got a rum deal from those folks in Tokyo and HK.
I'd still like to buy the auto valve from you.
Cheers, Don
PS It is well known, but little understood, that the required length of a fractionating column can be reduced from
the rule of thumb, 15-20 times the diameter, to 12 times the diameter, by cooling the column. Normally this is done with a
water jacket. You do it with an interior coil, but the principle is much the same. In both cases it is a total violation of
distillation theory (why Nixon hates it so much) but, it works anyway...
PPS. I ferment to c.14-16% sometimes 18% with glucose as feedstock. Assuming a 50% charge in boiler (good practice
and optimises surface area) I need to strip 150 liters an hour to get 20 liters of 80-85% an hour out. One drum will be 100
litres working capacity. (Filling more than half way reduces surface and slows down evaporation.)
Corn grits:
If there's ethanol in your gas tank, there's a 50-50 chance that it was filtered through corn grits,
using a technique developed at Purdue University. When the corn grits get saturated with water you just make more ethanol
out of them!Drying of ethanol refers to the process of removing the last 5 or 10% of water, in order to make anhydrous, or
"neat" alcohol.
vaporshttp://www.esb.ucp.pt/~bungah/downstre/vapordry.htm
Ladisch and his students at Purdue University
found that drying of ethanol vapors saved energy and was economic. The corn industry makes ethanol by fermentation and has
corn grits readily available. Passing the wet vapor over corn grits or other drying agents removes most of the water to yield
a product that is close enough to absolute ethanol. When the drying agent approaches exhaustion, it is regenerated by heating
and reused. Eventually the drying agent breaks down but has potential uses elsewhere in corn processing.
Source: Mike
Ladisch; ladisch@ecn.purdue.edu
You can order blueprints by post or by email HERE:
Contact Us
Theoretically, given enough time and effort, it is possible to distill to 97.2% where the solution is
an azeotropic concentration. For all practical purposes 95% is the limit. However, there is a conventionally distilled, commercially
available beverage alcohol that is 96% or 192 proof. . It's called "Polmos Alcohol". It is very
difficult to
find and about 30-35% more expensive than the 95% brands such as "Everclear".
Using calcium oxide
or dehydrated calcium
hydroxide it is possible to distill right to the brink of 100% purity but it is definitely not a
practical exercise.
It requires a significant amount of 90% alcohol to
produce a minor quantity of absolute alcohol
(which cannot remain absolute due to it's highly hygroscopic nature).
I have yet to locate a chart or formula that
makes
a percentage conversion to 60 F from a higher temperature. If one is available I would like to have it. But then,
the gauging of alcohol is also somewhat dependent on ambient atmospheric pressure. Personally, most of the time
I
use the SWAG method (scientific wild ass guess) and use a reduction of about 3% from a high normal room temperature to 60
F. That's close enough for
my purposes.
Wouldn't you think that production of the highest
possible purity
is probably a good idea in any alcohol that is to be used for beverage or medicinal purposes?
The higher the percentage,
the less chance of any
undesirable impurities but it is more difficult and costly to distill to the higher purity.
Don
Contact Us
| The distillation column is really 3 stills in one! |
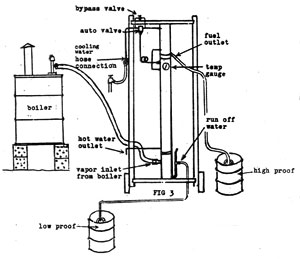
|
| My blueprints and written instructions show every small detail so you can build your own! |
Hi All,
Further to recent discussion about hydrometer correction for temperature
my standard h/b reference (CAMRA Home Brewing Guide) gives
following correction tables:
For
hydrometers calibrated @ 20 deg C
Temp (deg C) Correction
4-10 -2
11-17 -1
18-22 0
23-26 +1
27-29 +2
30-32 +3
33-35 +4
36-38 +5
39-41 +6
For hydrometers
calibrated @ 15.6 deg C
4-12
-1
13-18 0
19-23 +1
24-27 +2
28-30 +3
31-33 +4
34-36 +5
37-39 +6
40-41 +7
Ex: Liquid
has specific gravity 1085 @ 37 deg C using hydrometer calibrated @ 15.6 deg
C. sg corrected to 15.6 deg C
= 1085+6=1091.
Sg = specific gravity
Dick
Subject: wondering about sugar trees
Hello Robert,
I have about 50 acres of trees on my land and was wondering about maple trees....would it be a good source for sugar,
they sure make good maple syrup.
So I guess my question is: can I make alcohol fuel from this sugar? Seems to me that it would work, without all the mashing
of other sugar sources, (corn, apple or the like).
Thank You very much for your time.
...Marty B., Belfast, NY
Marty,
I pay something like $2.49 per pint for maple syrup at my local Trader Joe's, and think this a a good price for a great
product. I also am paying $2.49/gal for gasoline here in Calif. So this is the same price for 8 times less volume of syrup.
But To get your car to run on high proof, it would take you 10 gal. of syrup to make 1 gal of
high proof alcohol. It doesn't sound like a good idea to pay $199.20/gal for your fuel! ($2.49 x 8 x 10)
Find some day-old bakery wastes instead. Or read my page on molasses.
Robert Warren
PRINCIPLES OF OPERATION
|
 |
|
|
|
 |
|
We have just started an open forum newsgroup, called
Ethanol for Fuel!
This open discussion is
for people who are building their own still, making their own alcohol for fuel, and converting their cars to run on alcohol.
This list is for problem solving, sharing info about feedstocks, fermentation, distilling process, engine technology, and
making alcohol more efficiently. This is your public forum: anyone can subscribe, post, and read messages.
You can choose whether to receive messages in your email box, or just read them by logging in when you want to, so you
don't get swamped with messages.
http://www.topica.com/lists/robertwarren
Just post your email in this box below, and
you are a subscriber.
The following letters, on both sides of this page, were recieved before starting the newsgroup listed above.
Dear Robert
I did a thermodynamic analysis of the still. In order to get, as you claimed, even 5 gallons an hour (out
of 7 as per design), over an 8 hour period, of 160 proof, the following criteria would have to apply:
1. Assume that
the 9 hours does NOT include boil up time. If it does, you'd need a massive amount of heat, at least during the boil up phase.
2. At 16% in the mash, which is being generous, 40 gallons of 160 proof is 32 gallons of pure ethanol is 128 liters.
So, a wash containing 128 L of pure ethanol, would be about a c.800 L (200 gallon) volume, and so we know what
size the
boiler or boilers must be in aggregate. Let's say, 300-400 gallons,
I prefer to run a boiler half full, that range covers
50-75% full.
3. Boilup: it takes 1 kWH to bring 10L water to boil, so, we need 80 kWH for boilup. Trade off time vs energy.
1 kWH is 3400 BTU/Hr.
4. Takeoff: OK, 20 L an hour of 80% is 16 L ethanol and 4 L water. The 4 L water will consume 2.5
kWH and the 16 L ethanol, 3 kWH. That's because it takes 1 kW for every 1.6 L water and 1 kW for every 5.4 L ethanol. Latent
Heat of Vaporization. Water, 3X that of ethanol.
SO You needed a 5.5 to 6 kW heat source at takeoff. That will boil off
20 L of 160 proof an hour.
Consequences:
If 6 kWH (20,000 BTU) was your max heat input you'd have had a 13 hr boilup
time.
So you probably had more, and that's why you had to turn down the heat or face boil over.
A 'slobber box'
between boiler and the doubler would have helped prevent mash from reaching the column.
Your condenser needed to handle
6 kWH; your product was coming out hot, so the condenser could have been augmented with an external one and your product would
have been at ambient. Much safer when dealing with a high proof ethanol.
A conventional fractionating still (no reflux
head) same size as yours, packed with Raschig rings 6x6mm of porcelein, would usually be considered good for 3-4 kW so, you
were running at 50% over that, and that is
reasonably explained by the cooling coil through the packing.
Also you
didn't insulate the copper column so it is radiating heat like crazy and that would account for some of the difference. The
conventional way to go would be:
Dispense with the doubler and the cooling coil in packing -- make condenser bigger (more
coils, double coils, cold finger down middle).
Reduce heat to 3-4 kW, or else up the column size (length, diameter, or
both)
Measure the temperature at the top - a thermocouple and a digital thermometer is the way to go these days.
The
temperature will tell you when the product is all collected, as temperature will sit at 78-79, then start creeping up to 80-82,
which means you are taking off isopropyl, and some fusels -- wet socks smell in product.
That's when to quit for liquor.
The virtue of your design, for fuel ethanol, is IMHO, that it runs a bit faster and the predicted lower purity is irrelevant.
For liquor, the column needs to be run slower and the temperature gradient needs to be undisturbed by the intermittent
cooling, and the column absolutely needs to be well insulated.
Why dispense with the doubler? Because the double does
the job of 1 theoretical plate. The column, to your dimensions, has a good 15-20 theoretical plates depending on what the
packing is (NOI marbles) and so dispensing with 1 is ok.
If you keep the doubler, it works better if you have high proof
ethanol as the coolant instead of water from the start. Otherwise it takes some time for the doubler to be heated to boiling
point and to become saturated with
ethanol vapors, both of which conditions must be met before it does anything.
BTW,
the doubler will drive off methanol FASTER than ethanol, as methanol boils only at 60 C. So it won't trap methanol, acetaldehyde
or ethyl acetate. BUT, it probably does effectively trap some of the higher boiling
congeners like the fusel oils, acetic
acid etc.
well, good for 200
IN short, I find your prediction of 20 L/hr over 8 hours perfectly in line with theory,
assuming an adequate mash volume, boiler size commensurate, etc.
NOW: let's look at a 55 gal drum as boiler with maybe
40 gal (160 L) mash in there. This is about what you usually ran at demonstrations, right?
Boilup time @ 6 kW: 2.5 hours.
1.25 hrs @ 12 kW
Takeoff time: 25 liters of 80% will come off in c.1 hour maybe 65-70 minutes @ 6 kW. (This means you
turned down the 12 kW heat by half.)
Start to finish 2 hours and 15 minutes.
Is that about what happens? I have
to go reread chapter 2 again and see what you said.
The main criticism I would make is, the un-insulated column is just
wasting heat. The boiler and the condenser need to be well balanced but, letting so much heat escape is just a waste of energy,
and that's a shame in a biofuel
design.
On the other hand, if the 3" pipe was stainless steel and well insulated,
I'd recommend testing the thing at 3-4 kW before letting her rip at 6 kW.
And the through-put will drop by 33%. But you
will also need 33% less fuel to run your boiler.
Please take these as just friendly critique, which is how they are intended.
Regards, Don
PRINCIPLES OF OPERATION
I see there is a number of different opinions on proof figures which I think largely come about because of the different
books we have all read and sometimes inaccuracies in most hydrometers, methods, and inaccuracy in reading them.
Earlier
and the less researched books tend to quote a figure of 95% max because with distillation theory less understood and the more
inferior packings available in the past that generally was the maximum achievable.
There is a big difference between the
quality of hydrometers available in home brew stores and quality scientific hydrometers. To get a good genuine quality scientific
instrument in NZ and Australia you generally have to pay at least $100+. A lot of the hydrometers available in home brew shops
quite often have in accuracies of 3% and sometimes up to 5% or more. The old story you get what you pay for. No one is going
to sell you a Rolls Royce for the price of a Mini unless it fell off the back of a truck and even then the whole deal is totally
suspect anyway.
I know quite a number of otherwise quite reputable books claim that 95% is the maximum level for azoetropic
distillation but I believe from what I have read the top theoretical level attainable is 97.2% although I have never heard
of anyone attaining it as you need to use absolutely top notch equipment and everything must be perfect.( A bit like talking
about a frictionless system). Even then there is disagreement in the figures quoted with the figures quoted varying from 96.7%
at the bottom to 97.2 at the top. I certainly know 96.7% is obtainable as that is what Anchor Ethanol here, who produce hundreds
of thousands of gallons if not millions which is exported all over the world, used to produce at initially until they found
it was more economic to produce at the 96% level.
For all practical purposes it is 96 to 96.2% and for home distillation
very few of us get past 95% with a lot of us never getting past 90% due to the limitations of a lot of home distillation equipment.
Personally if your still is a reflux still and not a pot still and will not produce above the 90% level I would either
get out of it or upgrade it. As Des pointed out the other day and I have been trying to point out for some time if you are
only getting 70% you dont know exactly what is in that other 30%. In this case you then need to know what is in the water
or its composition that you dilute the alcohol with. At this point you then need to start learning something about water quality.
As my own town water supply is extremely hard due to going through 75 to 80 kilometres of concrete lined water pipes and mains
(9.2 to 9.5 pH) I choose to use distilled water I distill myself and what a difference.
Best regards, David
David--I
don't need or want 180 proof: unless I am running a racing engine. I get better mileage with 160 proof, as I am getting some
extra steam power for free from the water as it boils during the combustion cycle. You are talking about drinking it, I jsut
want to be able to drive to town and back with it, and have the extra power to pass the trucks in the way. --Robert
Rather
than spend mega-bucks on an accurate hydrometer, would it not be better to obtain a test solution of Ethyl alcohol from a
reliable source to calibrate ones own hydrometer? Do you know where we can get such a test solution?
Yes. Any laboratory
supply company sells 200 proof ethanol. I don't know the price per gallon but since there's no tax it should be reasonable.
Robert,
Thanks for the reply. I have a couple of questions/myths that you might help me with...
However,
the doubler (or thumper, as
some people call it) did most of the work of
removing the methanol as it was easily flash
cooled by the water in the doubler. Perhaps even
more important is the fact that the methanol is
easily soluble
in the water, and that it is the
fact that methanol is a more aggressive solvent
(in other words, faster to react
with other
elements) which makes it react quickly with the
water and therefore enter into a chemical mixture
or
compound.
How is it that the methanol forms a more positive bond with the water in the doubler, compared to the water
in the original wash? I can understand it initially being collected in there when the doubler is cold, but doesn't this heat
up after a while (because of the hot vapors passing through it), and then the methanol will start to come out of solution
as easily as it
did off the initial wash ? I haven't seen a doubler in action, but I'd figure that it would be operating
at around 82-85C after a while - warm enough to vaporize a fair bit of its methanol.
What sort of reaction is taking
place - what are the compounds it forms ? I had presumed that it would be fairly inert toward water. Why wouldn't these form
(& stay) in the initial wash too ?
Dear Tony,
It has to do with the fact that you have a flame or an electric
heating element under your cooking pot. The point of contact with the heat source may be well over 120 C or more, depending
on what you are using for fuel and how closely it is controlled. The beer or wash solution is where you are applying an excess
of heat, really, to boil the water which means you need to apply over 100 C heat, and both the ethanol and methanol are boiling
long before the water is. Actually, as you know from your graphs that you put up on your web site, the whole mix is boiling
at a lower temperature-according to the alcohol concentration you have. The water in the doubler does indeed heat up during
the process, but the methanol as a vapor is having to pass through a liquid barrier (same as the ethanol) but having more
free electron holes for the hydrogen in the water to adhere to, this happens very quickly. If your thumper or doubler is at
the bottom of the reflux column (rather than isolated as a separate, non-attached device like I have seen on some older style
stills, then in addition to the heat coming in from the bottom of the doubler, you also have cooling water coming down from
above in the reflux column. This higher the surface area of your packing materials inside the reflux column, the better this
works as you also have a constant heating effect going up and a cooling effect of water trickling down. Perhaps only half
of the ethanol vapors make it up to the top the first time through, more likely it is getting condensed somewhere midway up
the reflux column and falling down only to get caught by surface tension on the packing material and then re-heated by steam
rising from below.
If you look at the dynamics of a large commercial still, you will find that rather than random packing,
they have actual flat plates with holes in them so there is quite a baffling effect and lots of surface area for the up and
down reflux action to take place. These commercial stills are usually about 10 meters tall, and they run continuously, instead
of doing separate batches. The way it works is the beer is fed as a liquid by a pump into a port near the top of the reflux
column, and live steam is introduced at the bottom. The steam will also have an alcohol content, but you have quite a series
of waterfalls inside the 25 cm or larger column which is the reflux tower. Temperatures and flow rates are quite closely controlled,
and the column is usually insulated so as to eliminated any lack of control due to outside temperature conditions. So in a
large commercial still, you have the same process, only on a larger scale and there is very little chance that any methanol
will get through as it bonds so easily to the bottoms water. The other thing to remember is the amount o f methanol is a very
small percentage, overall compared to your ethanol content. Usually in a 5 % beer you will only have maybe .1 %, or probably
even lower than that. I don't have any of my reference books on alcohol anymore, I sent them all to Keith for use by the Biofuel's
group, so my figures could be off due to the fact that I am just trying to recall stuff I read about 25 yrs ago when I was
getting started in all this.
Now, the fusil oils get trapped for a different reason. They are also easily removed
by
the water which is cooling the vapors, but then it gets trapped by the surface tension of the water.
It floats
to the top, and gets poured off with the bottoms water.
I wonder if anyone has tried using surface tension modifiers
to get this happening a little better, non-ionic surfactants and the like? There must be a suitable one out there, which would
enhance this feature.
Now, here you are talking about something that is going to introduce another chemical or possibly
a bad taste to the mix.
Leave well enough alone, and if you do have any methanol, it will certainly come out in the charcoal
filtering and aging processes.
The charcoal will remove complex organic pollutants that you didn't even know you had.
The limitations of carbon filters are that they cant remove non-organic pollutants like lead, mercury, cadmium, and other
such metallic pollutants. However, carbon filters do a fairly nice job of removing chlorine and chlorine compounds, and that
is great, as some of the chlorine compounds can be fairly nasty in small doses.
This is maybe a separate issue completely,
but if you ever travel in Mexico or China, it is never safe to drink the water, but pretty much always safe to drink the beer.
The beer factories usually have pretty good water filtration, then the alcohol kills off the microbes. When I was living in
China last year, they had one kind of beer that had a big ass ugly beetle inside the bottle. A friend from the US came to
visit me, and when we were in the Chinese market, he bought a couple bottles of wine to take home (just for display, he didn't
want to drink it) called "Three snakes wine". There were three poisonous snakes curled up inside just one wine bottle. Now,
I know they put the worm in the Tequila bottle in Mexico, but perhaps those of you who are looking for an exotic flavor ought
to consider adding other live critters to your high proof if you are adventurous enough to go off exploring new taste territories.
I had presumed that they were retained here (more than in the wash) because
of the doublers lower temperature
than the wash (eg 90-96C), and hence lower volatility.
Yes, that is also true. It is both the lower temp and the reacting
with water.
cheers,
|
 |
 |
|
|
|



